Practical Understanding of Data Integrity in Pharma Industry
About Course
Data integrity is the foundation of a reliable Quality Management System (QMS) and is essential for safeguarding patient health and maintaining regulatory compliance within the pharmaceutical, biotech, and medical device industries.
This foundational course is designed to provide employees at all levels with a clear and practical understanding of the core principles of data integrity.
Who Should Enroll:
This course is specifically designed for all personnel working in GxP environments who handle data, including:
- Quality Assurance (QA) and Quality Control (QC) Personnel
- Manufacturing and Production Staff
- Information Technology (IT) and Computer System Validation (CSV) Specialists
- Regulatory Affairs Professionals
- R&D Scientists and Laboratory Analysts
- Managers and Supervisors Responsible for Data Oversight
Key Topics Covered:
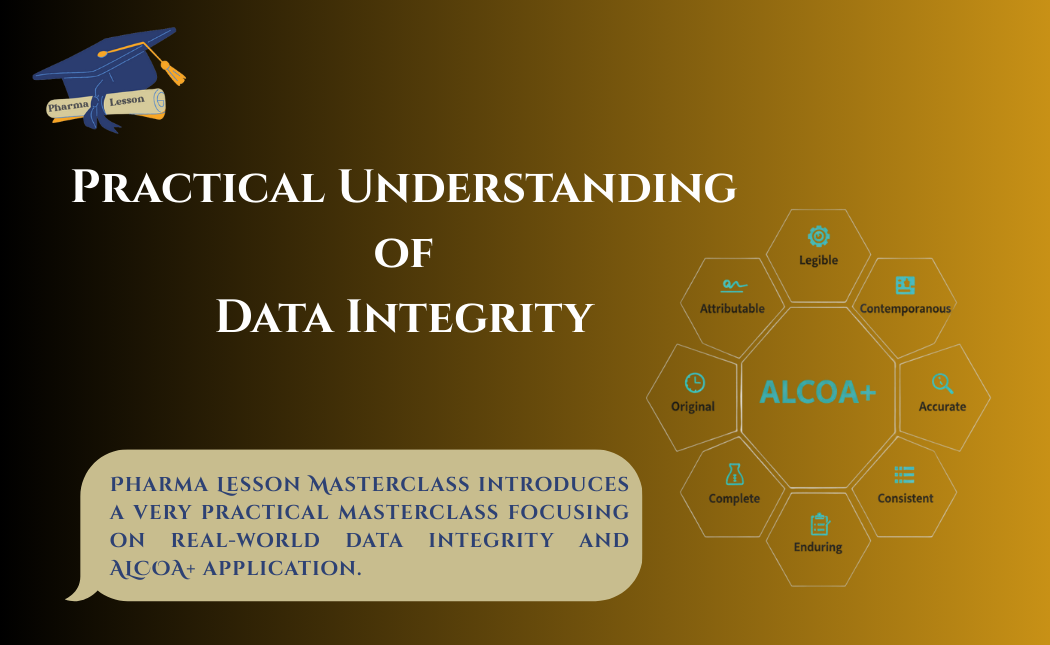
- ALCOA+ Principles
- Data Integrity by Design
- Data Governance Framework
- Data Integrity in Analytical Laboratories
- Data Integrity in Manufacturing Area
- Regulatory Guidelines on Data Integrity
- Data Integrity Checklist
- Data Integrity Risk Assessment
- Top 10 Data Integrity Traps
- Consequences of DI Issues
Course Format:
- Self-paced online modules
- Practical knowledge
- Lifetime access
- Quizzes & certification upon completion
Course Content
Module I
-
Introduction
-
ALCOA+ Principles
-
Data Integrity by Design
-
Data Governance Framework
-
Pre Assessment-1
Module II
Module III
Module IV
Module V
Final Assessment
Earn a certificate
Add this certificate to your resume to demonstrate your skills & increase your chances of getting noticed.

Student Ratings & Reviews
Better information
Awesome
Thanks for such informative course
This course is very much helpful for industrial pharmacists!

